If you have a parent with type 1 diabetes, your risk increases 3-4% compared to the general population’s 0.4%. With type 2 diabetes, having one affected parent raises your risk by 40%, and having both parents with the condition increases your risk to a startling 70%. These statistics from the American Diabetes Association highlight the powerful genetic connection to diabetes that shapes millions of families worldwide.
Even more revealing are twin studies: when one identical twin develops type 1 diabetes, the other has a 50% chance of developing it too – despite their identical genetic makeup. For type 2 diabetes, this jumps to 75% concordance. Yet these numbers tell us something profound – genetics alone doesn’t determine diabetes destiny, as even genetically identical individuals don’t always share the same outcome.
Perhaps most surprising is how genetic risk varies across populations. For instance, specific genetic variants associated with type 2 diabetes occur in approximately 40% of Europeans but 80% of certain East Asian populations. Yet prevalence rates don’t perfectly match these genetic differences, underscoring the complex interplay between genes and environment in diabetes development.
At IFitCenter, we recognize how critical it is to understand these genetic connections to diabetes. Today, we’ll explore the fascinating science behind diabetes genetics, what it means for your personal risk if you have family history, and most importantly, the evidence-based strategies that can help you potentially overcome genetic predisposition through targeted lifestyle modifications.
The Genetic Basis of Type 1 Diabetes
When we talk about type 1 diabetes being an autoimmune condition, what’s actually happening is that the body’s defense system – designed to fight off invaders like viruses and bacteria – mistakenly identifies the insulin-producing beta cells in the pancreas as enemies and systematically destroys them. This is similar to how a security system in your home might accidentally flag a family member as an intruder. But why does this “security breach” happen in the first place? Genetics plays a crucial role in this misidentification.
The HLA System: Your Immune System’s ID Badge
The most significant genetic factor in type 1 diabetes involves genes in the Human Leukocyte Antigen (HLA) system. Think of HLA genes as the ID badges your cells wear that tell your immune system, “I belong here.” These genes are responsible for producing proteins that sit on the surface of your cells and help your immune system distinguish between “self” (your own cells) and “non-self” (foreign invaders).
Two specific HLA variants – known as HLA-DR3 and HLA-DR4 – are strongly associated with type 1 diabetes risk. Approximately 90% of people with type 1 diabetes carry either DR3, DR4, or both, compared to about 40% of the general population. These variants essentially create a “faulty ID badge” that may confuse the immune system’s recognition process.
Type 1 diabetes results from immune-mediated destruction of insulin-producing pancreatic β-cells. This destruction happens gradually over time, with symptoms appearing only after approximately 80-90% of beta cells have been destroyed – like a slow-motion security breach that goes undetected until significant damage has occurred.
Family Risk Patterns: What the Research Shows
The genetic influence on type 1 diabetes becomes apparent when we examine family risk patterns. If you have no family history of type 1 diabetes, your risk is approximately 0.4% (about 1 in 250). However, this risk changes dramatically based on family connections:
- If your mother has type 1 diabetes, your risk increases to 1-4%
- If your father has type 1 diabetes, your risk rises slightly higher to 3-8%
- If both parents have type 1 diabetes, the risk jumps to approximately 30%
- If a sibling has type 1 diabetes, your risk is 6-10%
Perhaps the most revealing evidence comes from twin studies. For identical twins who share 100% of their genetic material, if one twin develops type 1 diabetes, the other twin has about a 50% chance of also developing the condition. This is significantly higher than the 5-10% concordance rate seen in non-identical twins, who share only about 50% of their genes.
These statistics demonstrate both the strong genetic component of type 1 diabetes and the fact that genetics alone isn’t the whole story. As Grant et al. note in their research: “Type 1 diabetes is the result of a complex interplay between predisposing genetic factors and environmental influences.”
Beyond HLA: Other Genetic Players
While the HLA system contributes about 50% of the genetic risk for type 1 diabetes, researchers have identified over 50 other genetic regions that also influence susceptibility. These include:
- Insulin gene (INS): Variations in this gene can affect how the insulin protein is recognized by the immune system, potentially triggering an autoimmune response. This is like having a slightly altered blueprint for insulin that makes it look suspicious to the immune system.
- PTPN22: This gene helps regulate T-cell activity (a key component of the immune response). Certain variants can lead to overactive immune responses, similar to having a security system set at too high a sensitivity level.
- CTLA4: This gene acts as a “brake” on the immune system. Some variants reduce this braking capacity, allowing immune responses to go unchecked – like a car with faulty brakes.
- IL2RA: Important for immune regulation, particularly of regulatory T-cells that help prevent autoimmunity.
Each of these genetic factors alone confers a relatively small risk, but in combination with HLA variants and environmental triggers, they create the conditions for type 1 diabetes to develop. It’s similar to how a combination of minor security vulnerabilities in a computer system can, together, create a major security risk.
In our IFitCenter blog, we have provided you with a completely free database about various diseases, which knowing about them will be very helpful in the prevention and control of diseases, especially diabetes. To access the first part of the materials related to diabetes, I recommend using the links below:
- diabetes complications
- difference between prediabetes and diabetes
- diabetes symptoms
- insulin resistance and diabetes
- can obesity cause diabetes
- low-sugar fruits for diabetics
- Superfoods for diabetes
The Multiple Hit Theory
Current understanding suggests that type 1 diabetes develops through a “multiple hit” process, requiring both genetic susceptibility and environmental triggers. This explains why even identical twins don’t always both develop the condition despite sharing identical genetic material.
Potential environmental triggers that might “activate” genetic susceptibility include:
- Viral infections (particularly enteroviruses)
- Early dietary exposures (e.g., cow’s milk proteins, gluten)
- Vitamin D deficiency
- Changes in gut microbiome composition
It’s like having a genetic predisposition to be a great pianist – you have the genes for musical ability, but without exposure to a piano and training (environmental factors), you might never become a pianist. Similarly, someone might have genetic risk factors for type 1 diabetes, but without certain environmental exposures, the condition might never develop.
“What fascinates me about type 1 diabetes genetics is the ‘threshold effect’ we observe in clinical practice. While HLA genes create susceptibility, they rarely cause disease without environmental triggers. This explains why we see families where siblings share identical high-risk HLA variants, yet only one develops diabetes. It’s a powerful reminder that genetic risk isn’t destiny, but rather one factor in a complex equation that includes viral exposure, vitamin D status, and even gut microbiome composition.”
Dr. Babak Jamalian, Family Physician.
Type 1 Diabetes Inheritance Patterns
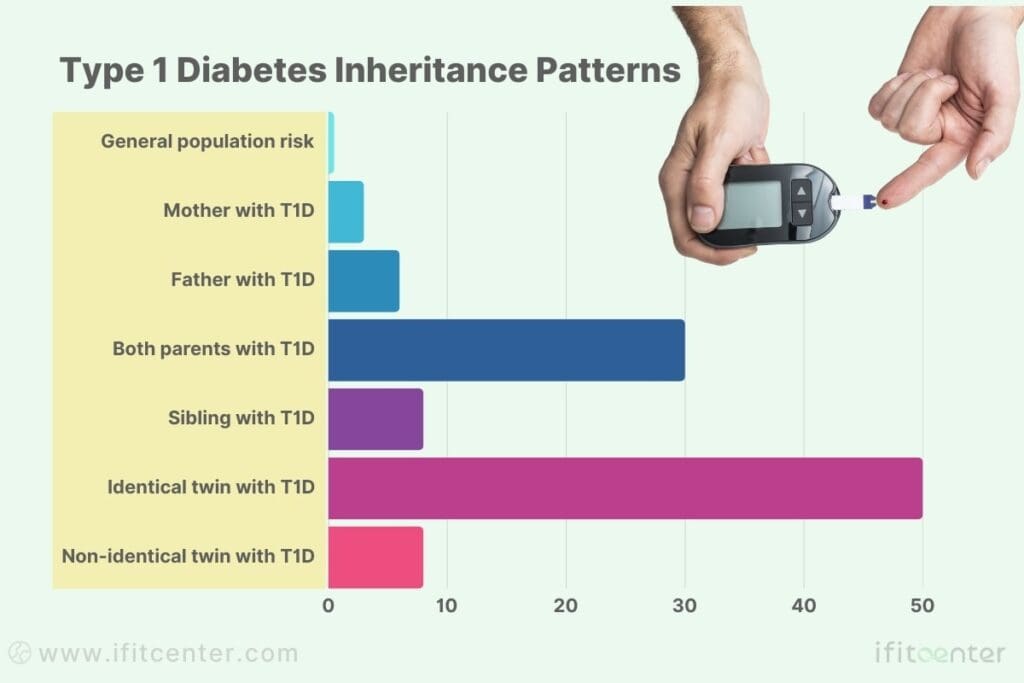
- General population risk: 0.4%
- Mother with T1D: 1-4% risk
- Father with T1D: 3-8% risk
- Both parents with T1D: ~30% risk
- Sibling with T1D: 6-10% risk
- Identical twin with T1D: ~50% risk
- Non-identical twin with T1D: 5-10% risk
Understanding these genetic factors doesn’t mean we can currently prevent type 1 diabetes, but this knowledge is invaluable for several reasons. It helps identify individuals who might benefit from early monitoring, allows for research into targeted interventions, and provides insights that may eventually lead to prevention strategies. Most importantly, it helps families understand that type 1 diabetes is not caused by anything they did wrong – it’s a complex interplay of genetics and environment beyond anyone’s control.
Genetic Factors in Type 2 Diabetes
While type 1 diabetes has a clear genetic component, type 2 diabetes also shows significant hereditary influence – though in a more complex pattern. Understanding these genetic factors helps explain why type 2 diabetes tends to run in families and varies across different populations.
Key Genetic Variants That Increase Risk
Research has identified several genetic variants associated with type 2 diabetes risk. Unlike type 1 diabetes, where a few genes have substantial impact, type 2 diabetes involves numerous genes each making a smaller contribution. Some of the most significant include:
- TCF7L2: The most widely replicated genetic risk factor for type 2 diabetes. This gene affects insulin secretion – think of it as influencing how efficiently your pancreas releases insulin. Variations in this gene can increase diabetes risk by 40-50%.
- KCNJ11: This gene regulates the potassium channels in pancreatic beta cells – essentially controlling part of the cellular machinery that releases insulin. It’s like having a factory with slightly different equipment that produces less efficiently.
- PPARG: This gene affects insulin sensitivity in fat cells. Certain variants make cells less responsive to insulin, similar to a cell phone with poor reception missing important signals.
- SLC30A8: Involved in the packaging and release of insulin in beta cells.
According to research by Prasad and Groop, “Over 400 different genetic signals for type 2 diabetes have been identified, but together they explain only about 20% of the heritability of the disease.” This indicates that while genetics plays a significant role, many genetic factors remain undiscovered, and environmental factors also contribute substantially.
How Genetics Affects Insulin Function
Genetic variants influence type 2 diabetes risk primarily in two ways:
- Insulin Production: Some genes affect the pancreas’s ability to produce insulin. These variants might reduce the number of insulin-producing beta cells or impair their function, similar to having fewer workers in a factory or workers who are less efficient.
- Insulin Sensitivity: Other genes affect how responsive your cells are to insulin. With decreased sensitivity, the body needs more insulin to achieve the same effect – like needing to turn up the volume to hear a muffled speaker.
The systematic review by Khawandanah explains that “family history of diabetes is a significant independent risk factor for type 2 diabetes, with those having a family history experiencing 2.77 times higher risk.” This risk increases further when both parents have diabetes, reaching 3.9-6.1 times higher risk compared to those without family history.
Ethnic Variations in Genetic Risk
One fascinating aspect of type 2 diabetes genetics is how risk varies across populations. According to the review by Nguyen et al., genetic risk factors for type 2 diabetes show significant variation across ethnic groups:
- The TCF7L2 gene variant occurs in approximately 40% of Europeans but shows different frequencies in other populations
- Pacific Islanders have 3.1 times higher diabetes prevalence compared to white populations
- Black individuals have 2.3 times higher prevalence
- Native Americans have 2.2 times higher prevalence
- Hispanic individuals have 2.0 times higher prevalence
These differences reflect both genetic variations and the interaction of genes with cultural and environmental factors. As noted in research analyzing populations worldwide, “Specific genetic variants associated with type 2 diabetes occur in approximately 40% of Europeans but up to 80% of certain East Asian populations.”
Genetics and Lifestyle: The Two-Way Street
Perhaps the most important understanding to emerge from genetic research is that type 2 diabetes results from the interaction between genetic predisposition and lifestyle factors. Even with high genetic risk, lifestyle choices can significantly affect whether diabetes develops.
In a landmark study examining lifestyle intervention in genetically predisposed individuals, researchers found that lifestyle changes reduced diabetes risk by approximately 58% in just three years – regardless of genetic risk profile. This demonstrates that while we can’t change our genetic makeup, we can influence how those genes express themselves.
This interplay works through several mechanisms:
- Epigenetic factors: Environmental influences can modify how genes are expressed without changing the genetic code itself – like having the same light switch but changing how often it’s turned on
- Metabolic load: Poor diet and sedentary lifestyle increase the demand for insulin, potentially overwhelming a genetically compromised system
- Fat distribution: Genetics influences where fat is stored, with visceral (abdominal) fat being particularly problematic for insulin resistance
“The many faces of diabetes: a disease with increasing heterogeneity,” genetics provides the predisposition, but lifestyle often provides the tipping point that leads to clinical diabetes.
“The genetic paradox of type 2 diabetes is something many patients misunderstand. While family history significantly increases risk, it’s not the genetics that have changed in recent decades as diabetes rates have soared. What’s changed is our environment’s interaction with those same genes. I’ve worked with numerous patients whose identical genetic profiles led to vastly different outcomes based on lifestyle choices. This isn’t about blame—it’s about recognizing that genetic predisposition provides valuable information about which environmental factors need our most diligent attention.”
Dr. Babak Jamalian, Family Physician.
Overcome Genetic Risk Through Targeted Weight Management
Genetics may increase your risk of diabetes, but targeted weight management can significantly reduce that risk. At iFitCenter, our medically supervised programs focus specifically on improving metabolic health and reducing diabetes risk through structured lifestyle modifications.
Our specialized services include:
- Personalized Metabolic Assessments tailored to your genetic risk profile.
- Customized Nutritional Guidance designed for better blood sugar control.
- Structured Weight Loss Plans focused on reducing insulin resistance.
- Regular Medical Monitoring to ensure continuous progress and adjustment.
Take control of your health today—your genes don’t have to determine your future.
Infographic: Genetic Risk Across Populations
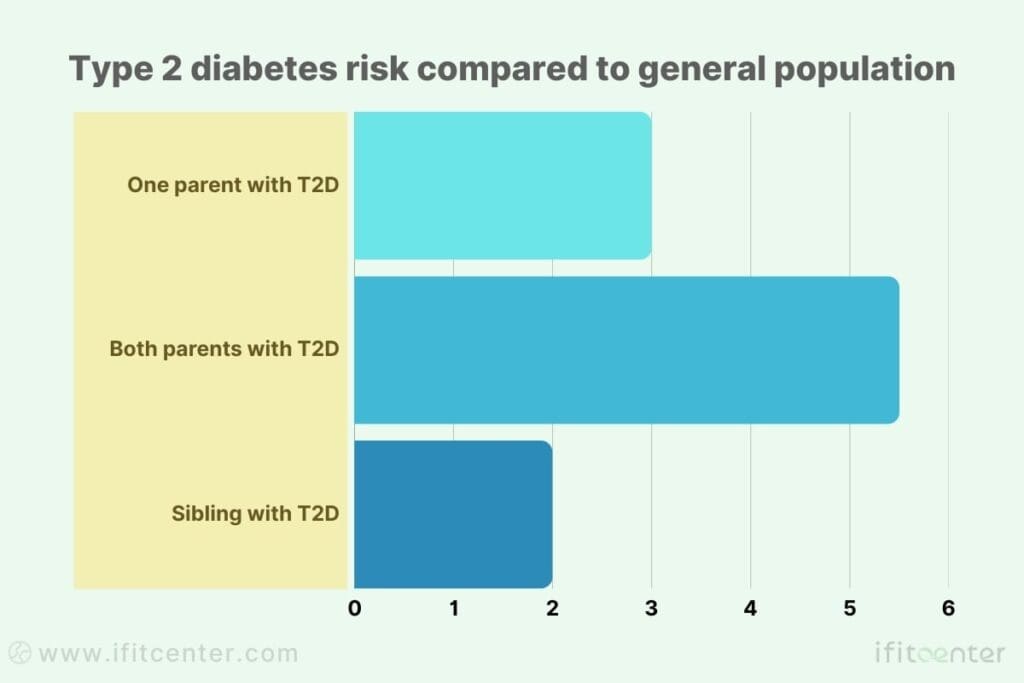
Type 2 diabetes risk compared to general population:
- One parent with T2D: 2-4 times increased risk
- Both parents with T2D: 5-6 times increased risk
- Sibling with T2D: 2-3 times increased risk
- Relative risk by ethnicity (compared to white populations):
- Pacific Islanders: 3.1x
- Black individuals: 2.3x
- Native Americans: 2.2x
- Hispanic individuals: 2.0x
- Multiracial individuals: 1.8x
Understanding the genetic factors in type 2 diabetes helps explain family patterns and population differences, but more importantly, it emphasizes that genetic risk doesn’t equal destiny. Even with strong genetic predisposition, lifestyle interventions remain effective at reducing diabetes risk. This knowledge doesn’t just help explain why diabetes develops – it provides the foundation for effective prevention strategies even for those with genetic vulnerability.
Understanding Genetic Testing for Diabetes Risk
With increased awareness of genetic factors in diabetes, many wonder if genetic testing can predict their risk. Here’s what you need to know about current testing options and their value.
In this section, we invite you, dear readers, to study the second part of the materials, which is related to diabetes, in the IFitCenter blog through the links below:
- how to test for diabetes
- is diabetes curable
- difference between prediabetic and diabetic
- diabetes explained in simple terms
- hba1c diabetes range
- superfoods for diabetes
- is diabetes genetic
Available Testing Options
Three main approaches exist for assessing genetic risk:
- HLA Typing: Tests for specific variants (HLA-DR3 and HLA-DR4) strongly linked to type 1 diabetes risk
- Polygenic Risk Scores: Examines multiple genetic variants to calculate overall risk
- Autoantibody Testing: Not genetic testing but often more useful; detects antibodies targeting insulin-producing cells
Most medical organizations don’t recommend routine genetic testing for diabetes prediction in the general population due to limitations in accuracy and utility.
When Testing Makes Sense
Genetic testing is most valuable in specific situations:
- Diagnosing unusual forms of diabetes (like MODY)
- Distinguishing between diabetes types when symptoms are atypical
- Research participation
- Family planning with strong history of type 1 diabetes
For most people concerned about diabetes risk, standard medical tests (blood glucose, HbA1c) combined with family history assessment provide more actionable information than genetic testing.
Testing Limitations
Important limitations of genetic testing include:
- Poor predictive accuracy (even identical twins with identical genes don’t always both develop diabetes)
- Inability to account for environmental triggers
- Expensive and often not covered by insurance
- Results can be difficult to translate into practical action
For type 1 diabetes risk assessment specifically, autoantibody testing often provides more practical information than genetic testing by identifying whether the autoimmune process has already begun.
Early Warning Signs for Those with Genetic Predisposition
For people with family history of diabetes, recognizing early warning signs can help identify the condition in its earliest, most manageable stages.
Type 1 Diabetes Early Indicators
Type 1 diabetes typically develops gradually, with several stages before symptoms become obvious:
- Autoantibody presence: The earliest sign, detectable through blood tests before symptoms appear
- Subtle glucose changes: Slight elevations in blood glucose or abnormal glucose tolerance tests
- Declining C-peptide: Indicating gradual loss of insulin production
These changes usually don’t cause noticeable symptoms but can be detected through specialized testing in high-risk individuals.
Type 2 Diabetes Warning Signs
For type 2 diabetes, early warning signs include:
- Prediabetes: Blood glucose levels above normal but below diabetic range
- Skin changes: Darkened patches (acanthosis nigricans) typically on neck, armpits or groin
- Metabolic changes: High triglycerides with low HDL cholesterol
- Increased waist circumference: Especially with normal BMI
- Subtle symptoms: Minor increases in thirst/urination, fatigue after carbohydrate-rich meals
Key Blood Markers to Monitor
If you have family history of diabetes, these measurements deserve regular monitoring:
- Fasting blood glucose: Ideally below 100 mg/dL
- Hemoglobin A1C: Reflects average blood glucose over 2-3 months; values between 5.7-6.4% indicate prediabetes
- Fasting insulin levels: Can reveal insulin resistance before glucose abnormalities appear
- Lipid profile: Especially triglycerides and HDL cholesterol
For those with genetic risk, detecting these changes early provides a significant advantage, as intervention at pre-diabetic stages is more effective than waiting until diabetes develops.
Evidence-Based Prevention Strategies for Genetically At-Risk Individuals
Understanding your genetic predisposition empowers you to take targeted actions that can dramatically reduce the likelihood of developing diabetes, even with high genetic risk.
Type 1 Diabetes Prevention Approaches
While type 1 diabetes prevention remains challenging, research suggests potential protective factors:
- Vitamin D supplementation: May help regulate immune function
- Omega-3 fatty acids: Shows potential for modulating autoimmune responses
- Infant feeding practices: Introducing gluten between 4-7 months (not earlier or later) may reduce risk
- Regular monitoring: For those with family history, participating in screening programs helps detect early stages
Type 2 Diabetes Prevention Strategies
For type 2 diabetes, strong evidence supports specific interventions:
- Weight management: Even modest weight loss (5-7% of body weight) reduces diabetes risk by approximately 58% in high-risk individuals
- Mediterranean diet: Reduces diabetes risk by about 30% compared to other diets
- Physical activity: Aim for 150 minutes weekly of moderate activity plus resistance training 2-3 times weekly
- Sleep optimization: Maintaining 7-8 hours of quality sleep helps regulate metabolism
- Stress management: Regular stress-reduction practices help control stress hormones that affect blood glucose
Research consistently shows that these lifestyle interventions significantly reduce diabetes risk even among those with high genetic risk scores, demonstrating that environment can substantially override genetic predisposition.
Practical Implementation Tips
- Consistency over intensity: Regular, moderate habits show better long-term outcomes than extreme approaches
- Combine approaches: Diet, exercise, sleep and stress management work synergistically
- Regular monitoring: Those with genetic risk benefit from more frequent health checks
- Early intervention: Acting at the first signs of metabolic changes brings better results than waiting for diabetes diagnosis
While genetic risk increases the importance of prevention, it doesn’t diminish the effectiveness of these strategies. With appropriate lifestyle modifications, even those with strong genetic predisposition can significantly reduce their risk of developing diabetes.
To access other content on the IFitCenter’s blog, you can use the following links:
References
- Madurapperumage Anuradha Erandathi, William Yu Chung Wang, Michael Mayo, Ching-Chi Lee. Comprehensive Factors for Predicting the Complications of Diabetes Mellitus: A Systematic Review. Curr Diabetes Rev. 2024;20(9):e040124225240. DOI: 10.2174/0115733998271863231116062601
- Matti Uusitupa, Tauseef A Khan, Effie Viguiliouk, Hana Kahleova, Angela A Rivellese, Kjeld Hermansen, Andreas Pfeiffer, Anastasia Thanopoulou, Jordi Salas-Salvadó, Ursula Schwab, John L Sievenpiper. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients. 2019;11(11):2611. DOI: 10.3390/nu11112611
- Karla Ivette Galaviz, Mary Beth Weber, Audrey Straus, Jeehea Sonya Haw, KM Venkat Narayan, Mohammed Kumail Ali. Global Diabetes Prevention Interventions: A Systematic Review and Network Meta-analysis of the Real-World Impact on Incidence, Weight, and Glucose. Diabetes Care. 2018;41(7):1526–1534. DOI: 10.2337/dc17-2222
- Lu, X., Xie, Q., Pan, X. et al. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Sig Transduct Target Ther 9, 262 (2024).
DOI: 10.1038/s41392-024-01951-9 - Ismail, L., Materwala, H., & Al Kaabi, J. (2021). Association of risk factors with type 2 diabetes: A systematic review. Computational and Structural Biotechnology Journal, 19, 1759-1785.
DOI: 10.1016/j.csbj.2021.03.003 - Ali, S., Hussain, R., Malik, R. A., Amin, R., & Tariq, M. N. (2024). Association of Obesity With Type 2 Diabetes Mellitus: A Hospital-Based Unmatched Case-Control Study. Cureus, 16(1), e52728. DOI: 10.7759/cureus.52728
- Harding, J. L., Wander, P. L., Zhang, X., Li, X., Karuranga, S., Chen, H., Sun, H., Xie, Y., Oram, R. A., Magliano, D. J., Zhou, Z., Jenkins, A. J., & Ma, R. C. W. (2022). The incidence of adult-onset type 1 diabetes: A systematic review from 32 countries and regions. Diabetes Care, 45(4), 994–1006.
DOI: 10.2337/dc21-1752 - Mobasseri, M., Shirmohammadi, M., Amiri, T., Vahed, N., Hosseini Fard, H., & Ghojazadeh, M. (2020). Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promotion Perspectives, 10(2), 98–115.
DOI: 10.34172/hpp.2020.18



